- LIB Recycling
A New Recycling Process for Spent Lithium-ion Battery Cathode Materials Based on Hydrothermal Method
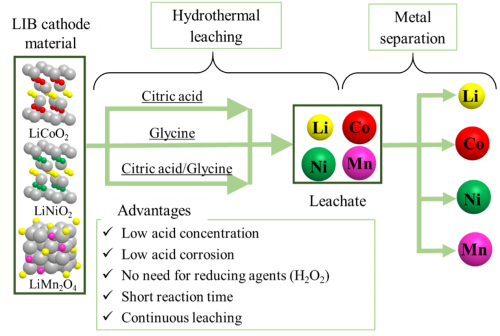
[Abstract] In recent years, there has been a growing interest in the use of renewable energy due to expectations of building a decarbonized society. Since the supply of renewable energy is unstable depending on the region and weather conditions, the use of energy storage media is essential. Lithium-ion batteries (LIBs) hold a large share of the energy storage media market, and demand and disposal of LIBs are expected to increase in the future. The current recycling process for LIBs involves an acid leaching process that dissolves valuable metals from the cathode material as ions, and a metal separation process that separately recovers multiple metals from the aqueous leachates. However, the acid leaching process requires the use of high concentrations of inorganic acid and hydrogen peroxide (H2O2) to promote the dissolution of metals, which places a heavy burden on the environment. In our laboratory, we have solved this problem by using high-temperature, high-pressure water as the reaction field for acid leaching and organic acid as the leaching agent, which has no potential environmental pollution at low concentrations. Currently, we are designing and fabricating a continuous acid leaching system and a continuous metal separation system, and are aiming for the complete continuous operation of the new LIB recycling process.
- Plastic Recycling
Development of “Liquid-Phase Hybrid Recycling Process” for Multilayer Plastic Film
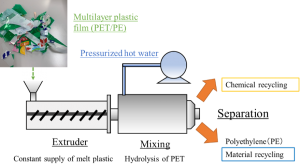
[Abstract] The environmental impact of waste plastic is increasing due to such factors as marine microplastics and the increase in CO2emissions from incineration, and expectations are rising for recycling technology that will enable recycling. In particular, plastic films used as packaging materials account for a high percentage of waste plastics; they are difficult to isolate due to the mixture of various components, and thus are mainly incinerated. In our laboratory, we are studying the recycling of plastic films made of polyethylene (PE) and polyester such as polyethylene terephthalate (PET), which have a high share in packaging plastics. The goal is to achieve “hybrid recycling”. The monomeric component of polyester can be recovered by hydrolysis in hydrothermal or subcritical water (liquid phase), and at the same time, PE, which has low reactivity, can be recovered undecomposed, so that each can be used as a raw material for plastic components. This research uses an extruder that continuously feeds molten plastic, and can achieve cleaner recycling using pressurized hot water without using environmentally hazardous solvents.
[Peer-reviewed papers]
[1] T. Aikawa, M. Watanabe, T.M. Aida, R.L. Smith Jr, Hydrothermal Leaching of LiCoO2 with Sulfuric Acid, Nitric Acid, and Citric Acid, Kagaku Kogaku Ronbunshu 43 (2017) 313-318.
[2] D. Azuma, T. Aikawa, Y. Hiraga, M. Watanabe, R.L. Smith Jr, Kinetic Study of Hydrothermal Leaching of Lithium Cobalt Oxide with Citric Acid, Kagaku Kogaku Ronbunshu 45 (2019) 147-157.
[3] Q. Zheng, M. Watanabe, Y. Iwatate, D. Azuma, K. Shibazaki, Y. Hiraga, A. Kishita, Y. Nakayasu, Hydrothermal Leaching of Ternary and Binary Lithium-ion Battery Cathode Materials with Citric Acid and the Kinetic Study, The Journal of Supercritical Fluids 165 (2020) 104990.
[4] Q. Zheng, K. Shibazaki, T. Ogawa, A. Kishita, Y. Hiraga, Y. Nakayasu, M. Watanabe, Continuous Hydrothermal Leaching of LiCoO2Cathode Materials by Using Citric Acid, Reaction Chemistry & Engineering 5 (2020) 2148-2154.
[5] K. Shibazaki, D. Azuma, M. Watanabe, A. Kishita, Y. Hiraga, H. Miyazaki, Hydrothermal organic acid leaching of positive electrode material of lithium-ion batteries, Kagaku Kogaku Ronbunshu 46 (2020) 167-175.
[6] Q. Zheng, K. Shibazaki, S. Hirama, Y. Iwatate, A. Kishita, Y. Hiraga, Y. Nakayasu, M. Watanabe, Glycine-Assisted Hydrothermal Leaching of LiCoO2/LiNiO2 Cathode Materials with High Efficiency and Negligible Acid Corrosion Employing Batch and Continuous Flow System, ACS Sustain. Chem. Eng. 9 (2021) 3246-3257.
[7] Q. Zheng, K. Shibazaki, T. Ogawa, A. Kishita, Y. Hiraga, M. Watanabe, Application of Hydrothermal Leaching Technology to Spent LIB Cathode Materials with Citric Acid Using Batch-type Device and Flow System, Journal of Chemical Engineering of Japan 54 (2021) 1-7.
[Patents]
[1] Separation of manganese and nickel, JP 2019-151440.
[2] Leaching method, separation method, and extractants for extraction of rare metals, JP 2019-166322.
[3] Synthetic method of manganese-citric acid complex, JP 2021-075442.
LIB Recycling
A New Recycling Process for Spent Lithium-ion Battery Cathode Materials Based on Hydrothermal Method
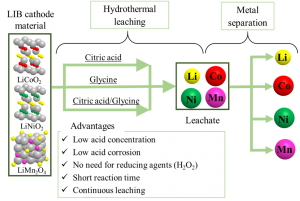
[Abstract] In recent years, there has been a growing interest in the use of renewable energy due to expectations of building a decarbonized society. Since the supply of renewable energy is unstable depending on the region and weather conditions, the use of energy storage media is essential. Lithium-ion batteries (LIBs) hold a large share of the energy storage media market, and demand and disposal of LIBs are expected to increase in the future. The current recycling process for LIBs involves an acid leaching process that dissolves valuable metals from the cathode material as ions, and a metal separation process that separately recovers multiple metals from the aqueous leachates. However, the acid leaching process requires the use of high concentrations of inorganic acid and hydrogen peroxide (H2O2) to promote the dissolution of metals, which places a heavy burden on the environment. In our laboratory, we have solved this problem by using high-temperature, high-pressure water as the reaction field for acid leaching and organic acid as the leaching agent, which has no potential environmental pollution at low concentrations. Currently, we are designing and fabricating a continuous acid leaching system and a continuous metal separation system, and are aiming for the complete continuous operation of the new LIB recycling process.
Plastic Recycling
Development of “Liquid-Phase Hybrid Recycling Process” for Multilayer Plastic Film
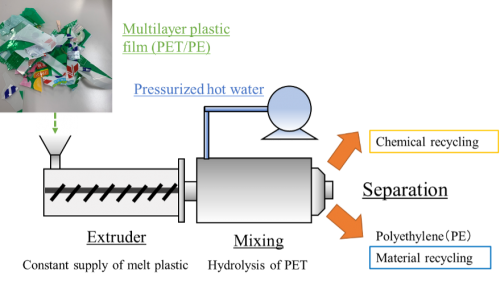
[Abstract] The environmental impact of waste plastic is increasing due to such factors as marine microplastics and the increase in CO2emissions from incineration, and expectations are rising for recycling technology that will enable recycling. In particular, plastic films used as packaging materials account for a high percentage of waste plastics; they are difficult to isolate due to the mixture of various components, and thus are mainly incinerated. In our laboratory, we are studying the recycling of plastic films made of polyethylene (PE) and polyester such as polyethylene terephthalate (PET), which have a high share in packaging plastics. The goal is to achieve “hybrid recycling”. The monomeric component of polyester can be recovered by hydrolysis in hydrothermal or subcritical water (liquid phase), and at the same time, PE, which has low reactivity, can be recovered undecomposed, so that each can be used as a raw material for plastic components. This research uses an extruder that continuously feeds molten plastic, and can achieve cleaner recycling using pressurized hot water without using environmentally hazardous solvents.